Also, like primary glaucoma, the two main types of secondary glaucoma are open angle and angle closure (also called narrow angle). But secondary glaucoma has many more subtypes.
The terms open angle and angle closure describe the basic structural issue within the eye that is disrupting aqueous flow. The names of the subtypes describe the underlying causes of those structural issues. Most of the subtypes can be either open angle or angle closure.
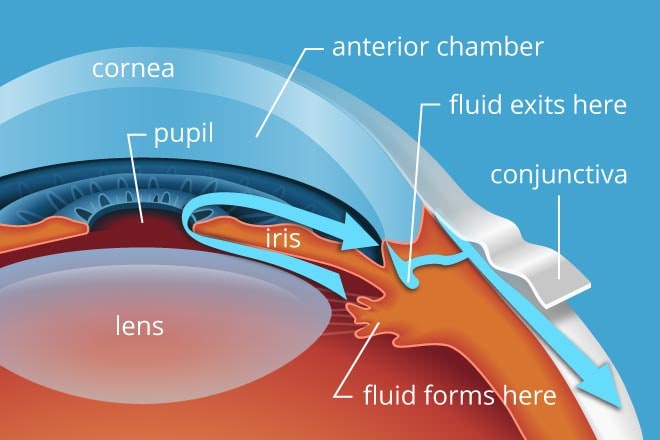
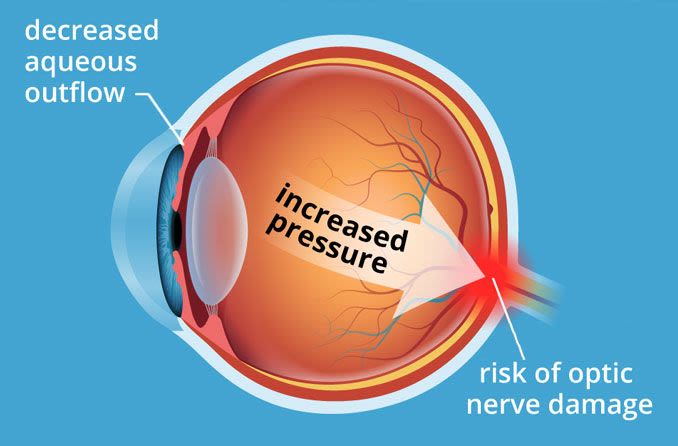
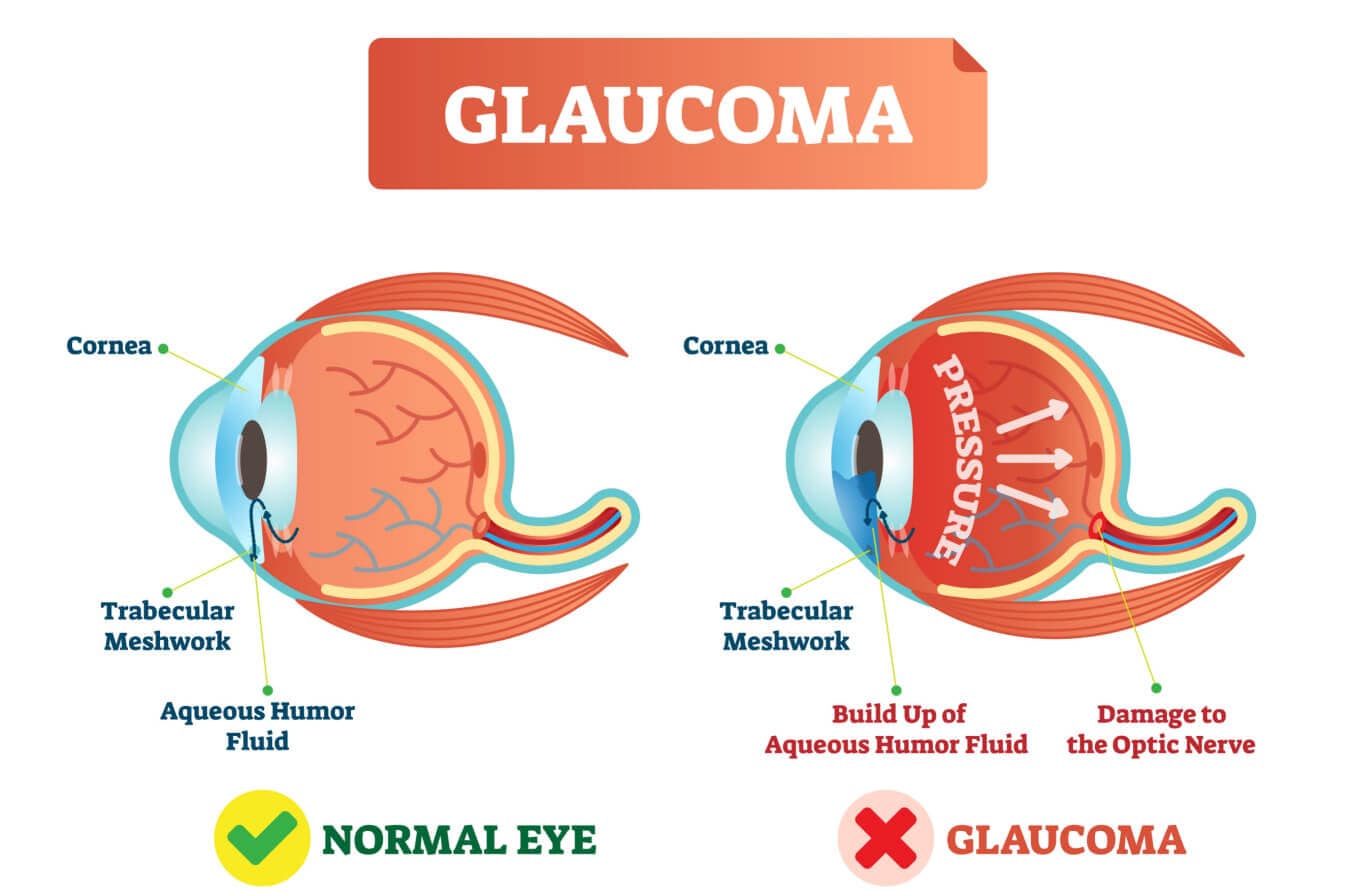
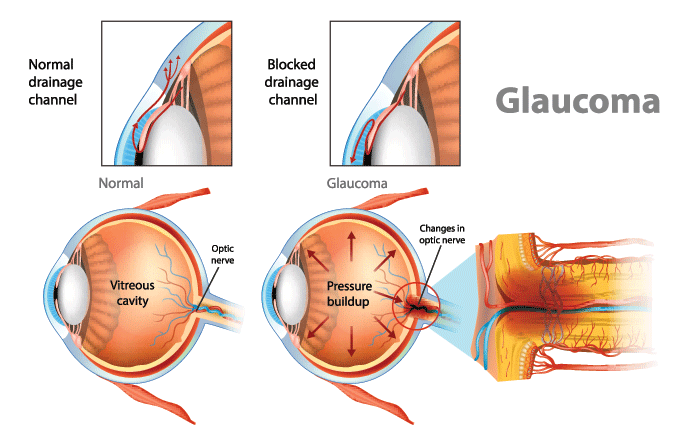
To keep our IOP balanced, the aqueous humor has to be able to flow freely along a specific path through and then out of the eye.
This path begins at the ciliary body, which produces the aqueous. From there, it flows along the underside of the iris, up through the pupil and then out through the drainage angle.
Beyond the angle, it filters through a tissue called the trabecular meshwork and other drainage structures. Then it is eventually reabsorbed into the body.
A smaller amount of fluid also leaves the eye through uveoscleral outflow. This fluid essentially seeps into and through the eye wall.
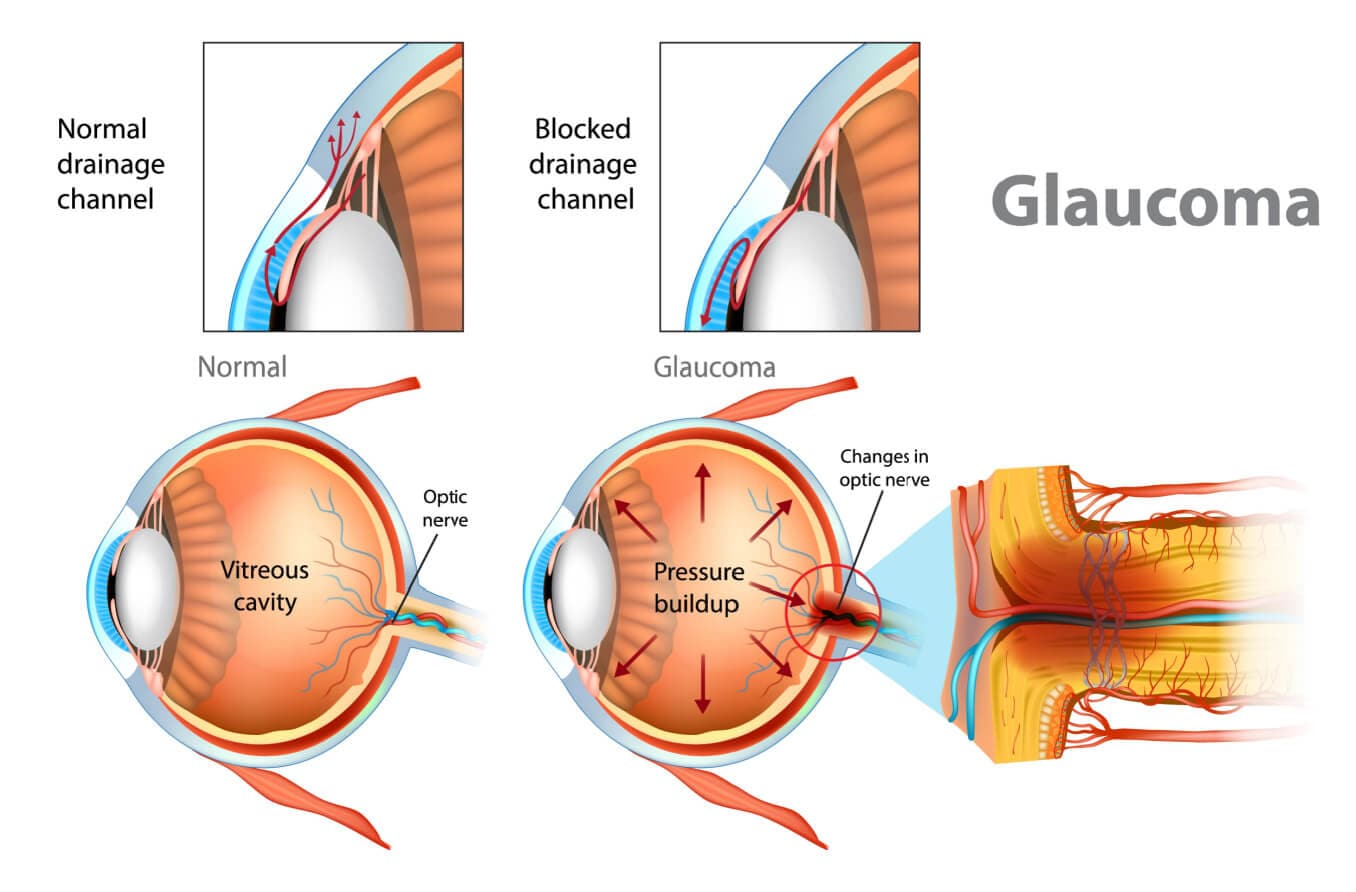
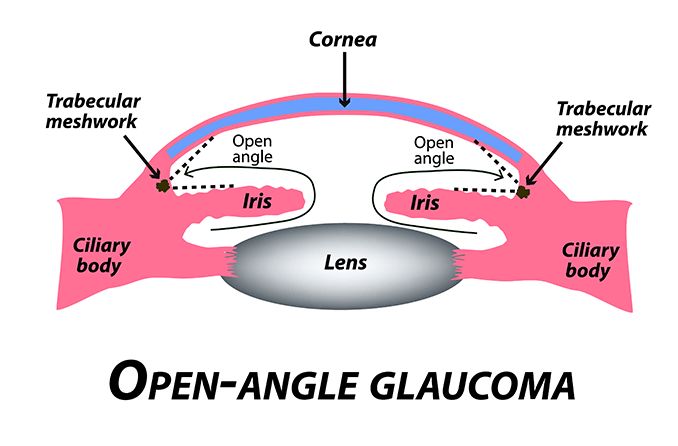
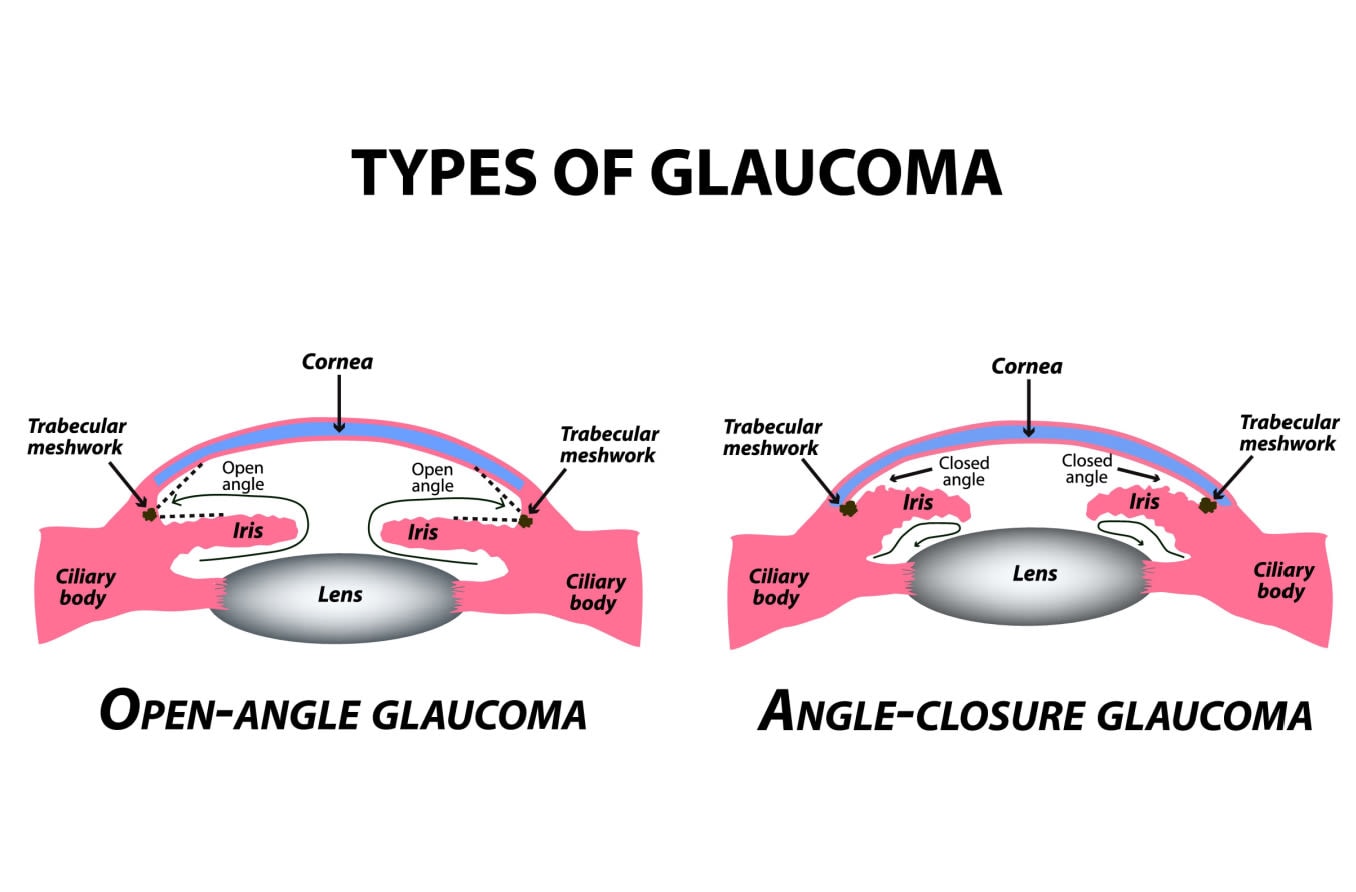
In open-angle forms, the aqueous flow disruption usually occurs in the trabecular meshwork.
In angle-closure forms, the aqueous can’t leave the eye because the iris is blocking the drainage angle.
LEARN MORE about glaucoma causes
Secondary open-angle glaucoma
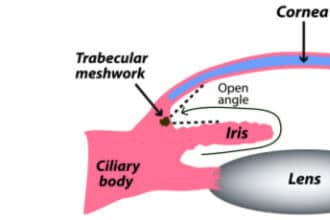
The drainage angle is a delta-shaped notch of space that surrounds the outside edge of the iris. It is formed by the iris on one side and the edge of the cornea on the other. This angle helps to funnel aqueous fluid into the trabecular meshwork.
When glaucoma develops in eyes that don’t have any obstruction in this notch of space, it is called open-angle. In most of these cases, outflow resistance in the trabecular meshwork causes IOP to increase.
The primary open-angle forms happen without any known cause. But in secondary open-angle glaucoma, something directly causes this resistance. Many conditions and outside factors can damage the trabecular meshwork or cause it to become clogged or inflamed.
Secondary angle-closure glaucoma
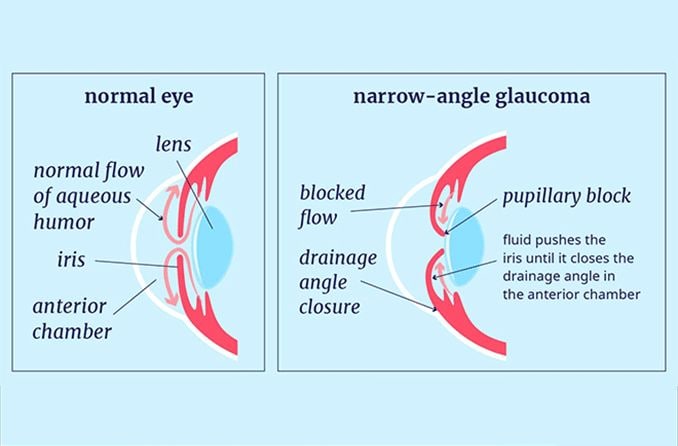
Angle closure occurs when the iris encroaches on or blocks the drainage angle. A narrow or closed angle will significantly slow down aqueous flow and cause IOP to spike.
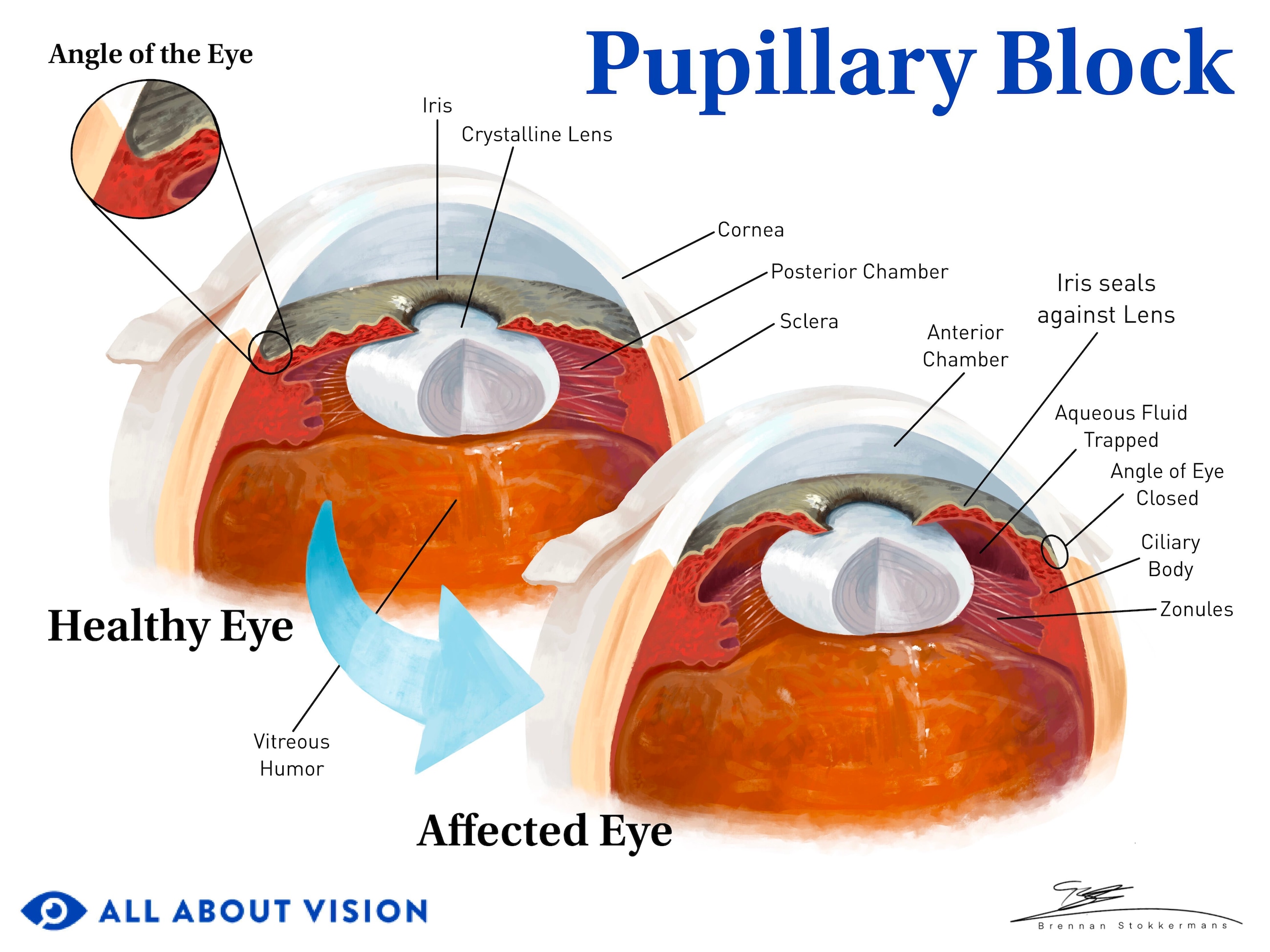
Often, pressure or crowding from behind the iris pushes it into the angle space. An enlarged or displaced lens can trap aqueous behind the iris if there isn’t enough space for it to flow through the pupil.
The pressure of the trapped aqueous causes the iris to bow forward and close the angle. This is called pupillary block.
While most secondary subtypes can be open- or closed-angle, secondary closed-angle glaucoma is less common. The subtypes that can be either/or usually begin with open angles and only progress to angle closure due to severe or chronic inflammation.
The main exceptions to this are closed angles caused by eye injuries and eye infections. A blow to the eye, even one that seems relatively minor, can dislocate the lens and quickly cause angle closure. Eye infections can also cause acute angle closure if the ciliary body and/or the iris are inflamed and swollen.
Pseudoexfoliative glaucoma (also called exfoliative glaucoma)
This form develops as a result of an age-related condition called pseudoexfoliation syndrome (PEX). In PEX, the body sheds protein molecules, producing a flakey, dandruff-like material. These flakes accumulate in organs throughout the body, including in the eyes.
Eventually, the eyes' drainage structures can become clogged or blocked by these flakes. When this happens, IOP increases, which can lead to pseudoexfoliative glaucoma (PXG).
Buildup of these flakes can also affect the iris and lens, limiting their ability to function. PEX can weaken the tiny fibers that hold the lens in place. As these fibers become looser, the lens can move forward and crowd the iris into the drainage angle. In these cases, PXG is a type of secondary angle-closure glaucoma.
PXG is the most common type of secondary glaucoma. However, it seems to affect some populations more than others. People with Scandinavian, Mediterranean, Arabic or Japanese backgrounds tend to have higher rates of both PEX and PXG.
Neovascular glaucoma
Certain conditions, such as diabetes, can significantly affect the blood supply to the eyes. The body may then try to correct this issue by growing new blood vessels. This is called neovascularization, or proliferative disease.
In people who have diabetes, this process is called proliferative diabetic retinopathy. Poor blood flow to the retina causes an overgrowth of new, but unhealthy, blood vessels on the retina and iris. Because the new blood vessels are weak and unhealthy, they also break and leak easily.
If left untreated, the overgrowth and leakage eventually clog the drainage pathway and cause IOP to increase.
Neovascularization of the iris can also cause an abnormal membrane to form in the space between the iris and trabecular meshwork. This membrane then pulls and binds the two together, closing off parts of the drainage angle. This is called neovascularization of the angle. These closed-off areas (called synechiae) can lead to secondary angle-closure glaucoma.
ICE syndrome-associated glaucoma
Iridocorneal endothelial (ICE) syndrome is a rare corneal tissue disorder. It can cause severe corneal swelling, iris deformation and secondary glaucoma. Though ICE itself is quite rare, this syndrome results in glaucoma in about 50% of cases.
In people who have ICE syndrome, abnormal cells from the cornea multiply and spread to other areas, including the drainage angle and iris.
As these cells move into the drainage angle, less and less fluid can pass through. And, since these cells are leaving the cornea, the cornea becomes both thinner and swollen, which can also increase IOP.
Sometimes, these two issues alone can damage the optic nerve. In these cases, glaucoma develops while the angle is still technically open. But often, ICE syndrome eventually leads to angle closure. This happens in a process that is very similar to angle closure in neovascular glaucoma.
The abnormal corneal cells form a continuous membrane as they spread through the eye. It connects the cornea, drainage angle, trabecular meshwork and iris. When it contracts, it pulls and stretches the iris toward the meshwork and binds them together in areas called synechiae. These synechiae close off the angle.
The swelling and stretching can also cause significant damage to the cornea and iris. There are three subtypes of ICE based on the degrees of this damage, but each subtype can lead to glaucoma.
Pigmentary glaucoma
This type of secondary glaucoma is caused by a condition called pigment dispersion syndrome (PDS). In PDS, tiny fragments of pigment from the iris come free and wash into the eye's drainage angle.
This tends to happen due to the relative placement in the eye of the iris and lens. If they are too close together, they can rub against each other. Certain movements, head postures and even accommodation can cause contact. The friction during this contact is what causes the iris pigment to come free.
Pigmentary glaucoma is relatively rare. It most commonly affects people who have myopia (nearsightedness) — usually white males. Myopic eyes tend to be longer than average from front to back. In some cases, this extra length may allow the iris to bow backward too far and touch the lens.
Traumatic glaucoma
This form can develop when trauma to the eye, such as an injury or surgery, causes increased IOP. Both blunt-force trauma (like being hit in the eye) and penetrating trauma (when a foreign object punctures the eye) can lead to traumatic glaucoma (TG).
TG can be either open- or closed-angle, depending on which tissues and structures are affected. For example, the injury could displace the lens and/or iris and disrupt fluid movement. This could lead to secondary angle-closure glaucoma. An injury can also damage the eye’s drainage structures or cause inflammation that impairs them.
Another common type of TG is called angle-recession glaucoma. The force of blunt trauma can drive the drainage angle backward, tearing tissues in the ciliary body and drainage structures. As the torn tissues heal and form scars, less and less aqueous can flow through.
Penetrating eye trauma can also trigger an autoimmune reaction in some people. When this happens, inflammatory cells can clog the eye’s drainage structures and lead to increased IOP. In the rare cases of secondary glaucoma following eye surgery, this immune response tends to be the cause.
It’s important to note that TG can have an early onset or a late onset. Some injuries can cause IOP to increase right away. But it is very common for TG to develop 10 or more years after an injury.
For this reason, it’s very important for anyone who has had an eye injury to get yearly comprehensive eye exams. TG can develop well after an eye injury is healed and forgotten — even those that seem trivial.
Uveitic glaucoma
The uvea is a continuous tract of tissue that makes up three very important parts of the eye: the iris, ciliary body and choroid. When any of these parts become inflamed or infected, it’s called uveitis.
Uveitis can be divided into four types, depending on the area(s) affected: anterior, intermediate, posterior and diffuse. The most common type, anterior uveitis, involves the iris and ciliary body. This is the type most commonly associated with uveitic glaucoma.
However, all types of uveitis can disrupt aqueous flow in one or more ways and lead to glaucoma. The open-angle forms are typically due to inflammatory cells and their debris released into the front of the eye. They can clog and even permanently damage the eye’s drainage structures.
Uveitis can also lead to secondary angle-closure glaucoma. The severe inflammation can trigger neovascularization and/or the formation of synechiae. The synechiae may form in the angle, closing it off, or between the iris and lens, leading to pupillary block.
The most common treatment for uveitis is corticosteroid eye drops, but steroids are known to worsen glaucoma. This, combined with the complex ways uveitis can increase IOP, makes uveitic glaucoma one of the most difficult to treat.
Posner-Schlossman syndrome (PSS) uveitic glaucoma
PSS is a relatively rare condition in which a person has recurring attacks of anterior uveitis. IOP spikes during these acute episodes, and, over time, this can lead to secondary glaucoma.
Also called glaucomatocyclitic crisis, PSS uveitis is different from other types of uveitis in a few important ways:
1 - It’s recurrent and unpredictable. Recurrent uveitis is typically related to an autoimmune issue, but this is not the case with PSS. There are no known underlying causes or flare-up triggers for PSS.
Each repeated episode of acute uveitis may last less than a day, or it may last for months. They may recur every few weeks or months, or there may be years between them.
2 - Its symptoms are milder. Acute anterior uveitis usually causes significant eye pain, redness, light sensitivity and blurred vision. With PSS, inflammation and its typical symptoms are minimal.
Most patients only notice minor eye discomfort and/or blurry vision. Many don’t have symptoms at all.
3 - It raises IOP drastically. All types of uveitis can have a dramatic impact on IOP. Usually, the increase in eye pressure it causes is directly related to the degree of inflammation in the eye.
But with PSS, eye pressure during an attack can be double or even quadruple the normal range, even without inflammation. In most cases, IOP does go back to normal between attacks. However, the spikes in eye pressure can cause permanent optic nerve damage.
There is not much research to show how rare PSS truly is or how often it causes glaucoma. But the studies that do exist estimate that PSS affects around two people per 100,000, and about a quarter of those people develop glaucoma.
Steroid-induced glaucoma
This secondary form can develop when IOP increases as a response to corticosteroids. In some people, steroids can cause physical changes within the eyes’ drainage structures and increase abnormal cell debris. These problems raise IOP and can lead to glaucoma.
People whose eyes are affected this way are called steroid responders. Experts estimate that 30%-40% of people are high or moderate responders. Steroid responders are at higher risk, but anyone can develop steroid-induced glaucoma. This is because even a low responsiveness may increase IOP enough to damage the optic nerve.
Other risk factors for steroid-induced glaucoma include:
A family history of glaucoma
Diabetes
High myopia
Connective tissue diseases
Keratoplasty (corneal transplant)
Age (6 years old or younger)
All types of steroids can have this effect, whether they are prescription or OTC. Steroid eye drops and eye ointments tend to cause the strongest responses. However, pills, nasal sprays and injections can also raise IOP.
Topiramate-induced glaucoma
Another medication that can increase IOP is topiramate. Doctors prescribe topiramate to treat seizure disorders and to prevent migraine headaches. It is also used to treat neuropathic pain and several mental and behavioral disorders.
A possible side effect of this drug is swelling of the choroid, the middle layer of the eye wall. This swelling can squeeze the back of the eye, forcing the lens and iris forward. In very rare cases, this can cause secondary angle-closure glaucoma.
The forward movement of the lens also causes acute myopia (sudden nearsightedness). If you notice a blurring of your vision after starting topiramate, see an eye doctor immediately.
Childhood secondary glaucoma
Secondary glaucoma can develop in children for many of the same reasons it develops in adults. For example, eye injuries, infections and steroid eye drops can all raise IOP in children and potentially lead to optic nerve damage.
The most common causes of secondary childhood glaucoma vary among different populations. But the top two causes tend to be eye injury and eye surgery. The most common type to develop due to eye surgery is called aphakic glaucoma. It can develop in children with pediatric cataracts after they have cataract surgery.
Retinopathy of prematurity is another eye disease that increases a child’s risk. With this condition, glaucoma can develop as a result of the retinopathy itself or as a complication after surgery to treat it.
Childhood glaucoma can also develop secondarily to genetic conditions, including:
Many other genetic conditions are also associated with glaucoma.