What is glaucoma and how does it affect your vision?
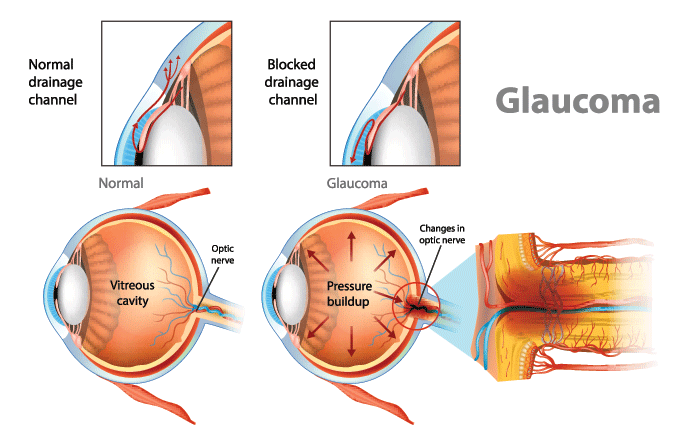
Glaucoma is a group of eye diseases that damage the optic nerve, which is the pathway that carries all the information from your eye to your brain about what you see. Glaucoma needs to be treated or you can permanently lose some or all of your vision.
If you’ve been diagnosed with glaucoma, it’s normal to feel concerned. The good news is that treatment is usually very effective at preventing future vision loss. You can help protect your eyesight by following your eye doctor’s treatment plan and going to regular checkups.
Does glaucoma cause symptoms?
Many people with glaucoma don’t have symptoms until they notice blind spots in their side vision (called peripheral vision). If glaucoma isn’t treated, the damage will spread to more of your peripheral vision, and eventually, the center of your view.
Vision loss can happen slowly, and it’s usually hard to notice until it’s already advanced. Half of people with the most common kind of glaucoma don’t know they have it.
That’s why routine comprehensive eye exams are so important, even when your eyesight seems normal. Eye doctors can spot the early signs of glaucoma and start treatment before it harms more of your eyesight.
Glaucoma can cause other symptoms
There’s a less common type of glaucoma that can cause eye symptoms, like pain, redness and blurry vision.
Sometimes, these symptoms are severe and happen very quickly. This is a vision-threatening emergency that needs immediate treatment.
Why does glaucoma happen?
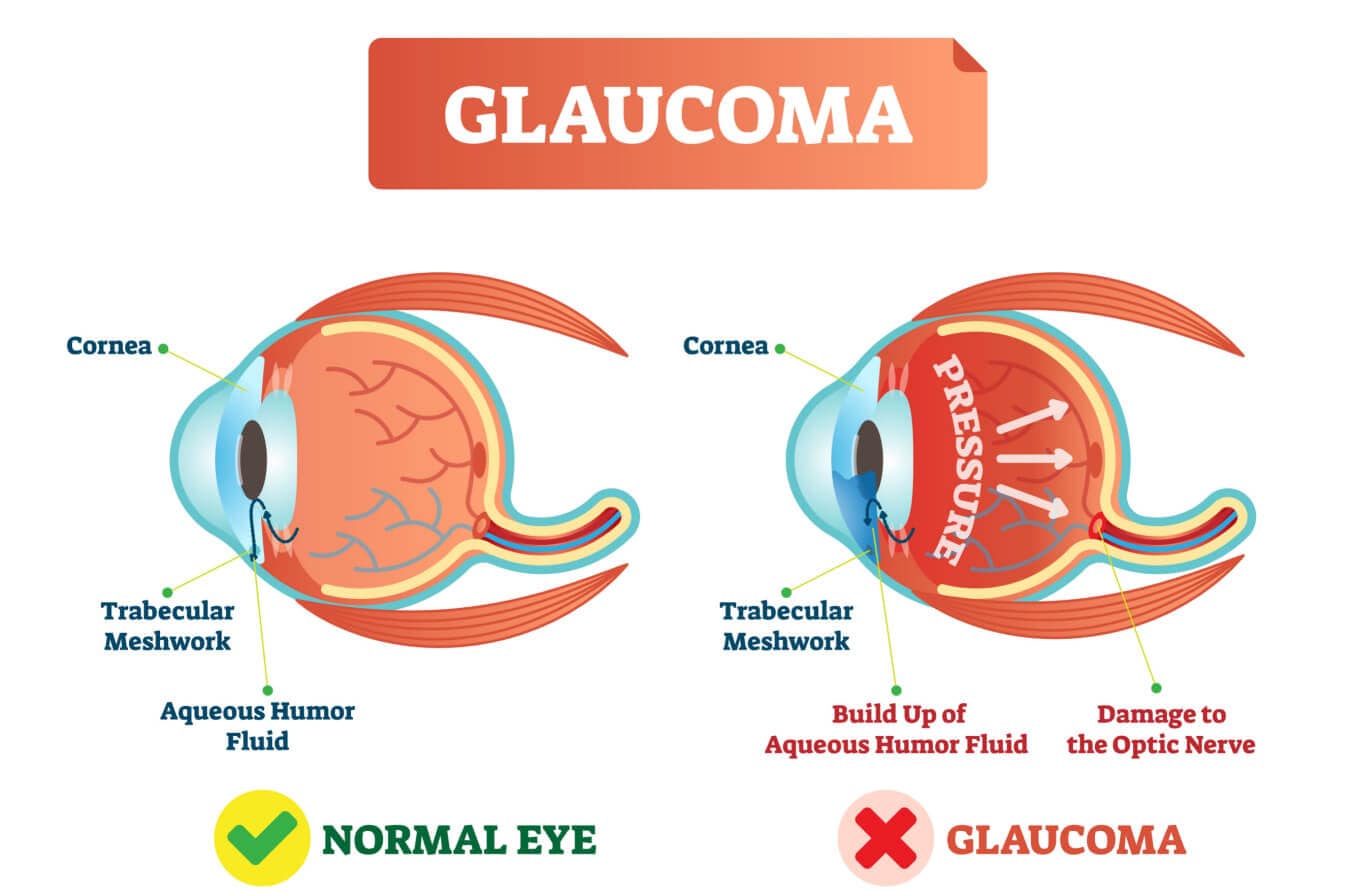
Your eye is constantly making a watery fluid called aqueous humor to fill and nourish the front area near your pupil. As new aqueous fluid comes in, the old fluid drains out.
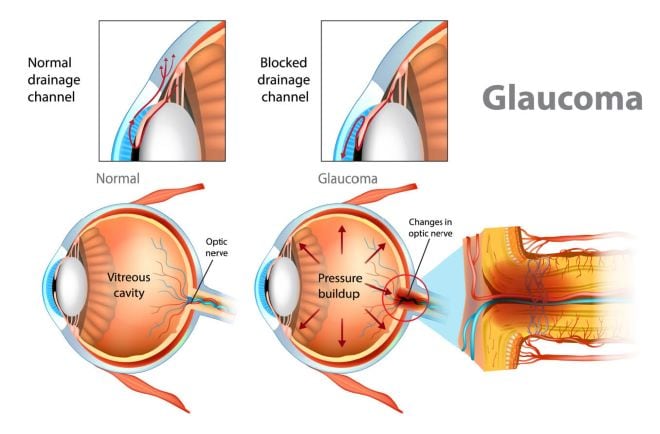
In most cases of glaucoma, the fluid doesn’t drain well, so it builds up inside the eye. This raises the pressure in your eye (doctors call this intraocular pressure or IOP).
Your optic nerve and eyesight can be damaged when your eye pressure is too high. An eye doctor can lower it to help prevent future vision loss.
What are the different types of glaucoma?
The two main types of glaucoma are open-angle and angle-closure, but there are other ways eye doctors can classify it.
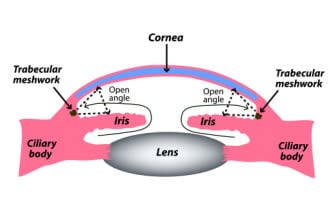
Open-angle glaucoma: The most common form
Near the spot where the colored part of your eye (iris) meets the clear front layer of your eye (cornea), there’s a small space called the drainage angle. Most of the aqueous fluid in your eye drains out through this angle.
Open-angle glaucoma is when the angle is not blocked, but the fluid still doesn’t drain out very well. This makes your eye pressure go up over time.
Open-angle glaucoma is usually painless and can develop slowly, sometimes over several years. Most people won’t have any symptoms until they start to notice blind spots. The vision loss can’t be reversed, but treatment can help stop future damage.
The most common type is primary open-angle glaucoma (POAG). “Primary” means it happens without a known cause.
Angle-closure glaucoma: A less common emergency
Angle-closure glaucoma is when the angle is blocked. The fluid can’t drain out of the eye, so eye pressure rises. This can happen slowly (chronic) or suddenly (acute).
Some people with the long-term type may get red eyes, headaches or other symptoms. Sometimes, the symptoms get better with sleep.
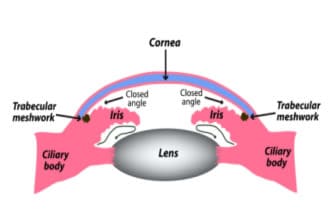
Narrow-angle / Angle-closure glaucoma
What is acute angle-closure glaucoma?
Acute angle-closure glaucoma (the sudden type) is an emergency that needs to be treated right away. Without treatment, you can start to lose vision in as little as two or three hours.
This type of glaucoma causes symptoms that come on very quickly. They can include:
- Intense eye pain
- Headache
- Blurry vision
- Seeing rainbow-colored halos around lights
- Feeling sick to your stomach or throwing up
Normal-tension glaucoma: Eye pressure isn’t high
Some people have glaucoma, even though their eye pressure is in the normal range. While the measurement is normal, it’s still too high for that particular person’s optic nerve.
This is a type of open-angle glaucoma called normal-tension glaucoma. Someone with this type still needs to be monitored and treated like someone with high eye pressure does.
Congenital glaucoma: Present at birth
Around 1 in 10,000 babies is born with glaucoma. This type (congenital glaucoma) is often treated with surgery that can save most or all of a newborn’s eyesight.
You can usually see signs that a baby has congenital glaucoma. Their eyes may look:
- Cloudy
- Bigger than normal
- Teary
- Sensitive to light
Secondary glaucoma: Caused by something else
Secondary glaucoma is caused by a separate problem. This can lead to open-angle or angle-closure glaucoma at any age, depending on the cause.
Many different problems can cause secondary glaucoma, such as:
- Other eye problems or health conditions
- Injuries or infections
- Surgery complications
- Side effects of medication
Are you at a higher risk for glaucoma?
Some people are more likely to develop glaucoma. Your risk is higher if:
- You are over 60 years old.
- You are of African, Hispanic, Asian or Inuit descent.
- Glaucoma runs in your family.
- You have diabetes or high blood pressure.
- You’ve used a steroid medicine for long periods.
- You’ve had an eye injury or eye surgery before.
Having one or more of these risk factors doesn’t mean you’ll definitely get glaucoma, but it does increase your chances. Your eye doctor might suggest getting eye exams more often so they can watch for any eye changes.
How do eye doctors diagnose glaucoma?
Eye doctors diagnose glaucoma with a combination of tests during an eye exam. The tests let them check your eye pressure, optic nerve, peripheral vision and other important factors.
Measuring your eye pressure
Your eye doctor will use a tonometry test to measure the pressure inside your eye.
Many people know this as the “puff-of-air” test in an eye exam, but that’s only one of three main types of tonometry. You might have one of the other types during a glaucoma exam because they’re more accurate than the air-puff test.
During the test, your eye doctor will numb your eyes with eye drops, then gently touch a small handheld device to the front of your eye to measure pressure.
What eye pressure measurement is too high?
A normal eye pressure measurement is between 10 and 21 mmHg, so most eye doctors consider anything over 21 mmHg to be high eye pressure.
“mmHg” stands for millimeters of mercury, which is the standard measurement for pressure.
Can you have high eye pressure without glaucoma?
Yes. High eye pressure and glaucoma are two separate things.
You can have high eye pressure without having glaucoma, and you can also have glaucoma, even when your eye pressure is normal.
People who have high eye pressure (or certain other changes) but no optic nerve damage are called “glaucoma suspects.” They don’t have glaucoma and may never develop it, but they still need regular checkups in case something changes.
Checking your optic nerve for damage
An eye doctor will check the optic nerve inside your eye for any changes that could be related to glaucoma. They may look through a magnifying tool or use a separate device that takes pictures or measurements.
The doctor might use eye drops to dilate (widen) your pupils so they can get a better view inside your eye. Dilating eye drops make your vision blurry for a few hours, so it helps to have a ride home arranged ahead of time.
Testing your field of vision
Your eye doctor may use one or more kinds of visual field testing to check whether glaucoma has affected your peripheral vision.
During the test, your doctor will ask you to look straight ahead and let them know as soon as you see something along the sides of your vision. The results create a “map” of your eyesight by showing the doctor if and where you have blind spots, which can be signs of glaucoma.
Examining the front of your eye
Eye doctors can check the parts of your eye that can slow down fluid drainage and raise your eye pressure. They might examine:
- The drainage angle – A special contact lens is held against your eye to see if the angle is open or blocked, along with other features. This test is called gonioscopy.
- The thickness of your cornea – A quick test called pachymetry is used to measure how thick each cornea is. Thin or thick corneas can make eye pressure readings less accurate, and thinner corneas may raise the risk for glaucoma.
How is glaucoma treated and managed?
Eye doctors help manage and treat glaucoma by lowering the pressure in your eye. This can involve eye drops, pills or special procedures.
Some people need two or more kinds of glaucoma treatment to keep their eyes healthy.
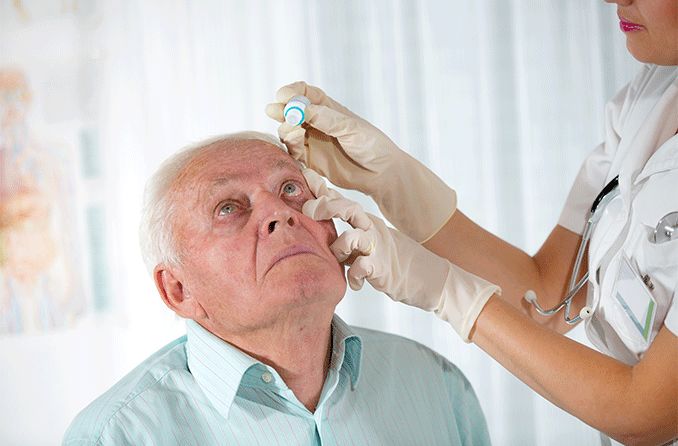
Prescription eye drops to lower eye pressure
Prescription eye drops are the most common treatment for glaucoma. Many times, they’re also the first treatment an eye doctor will try, especially for primary open-angle glaucoma.
There are many different kinds of glaucoma eye drops, but they’re all designed to help lower the pressure inside your eye.
Sometimes, the eye drops cause side effects in other parts of your body. If this happens, it’s important to tell your eye doctor so they can adjust your treatment or try other eye drops that might work better for you.
Oral medicine to lower eye pressure
An eye doctor may prescribe pills to help lower your eye pressure. They usually only recommend this if eye drops don’t help enough on their own.
These pills can also cause side effects, so tell your doctor if you notice anything unusual.
Laser procedures to improve drainage
Laser treatments are blade-free procedures that can help fluid drain more easily and lower your eye pressure. Two common kinds of laser surgery are:
- Laser trabeculoplasty – The laser makes an opening in your eye’s mesh-like drainage system, so fluid can flow out more easily. This is usually used for open-angle glaucoma.
- Laser peripheral iridotomy – The laser makes a small hole in the iris to help fluid flow more easily inside your eye. This is used for angle-closure glaucoma.
An ophthalmologist usually performs laser glaucoma surgery, but specially trained optometrists can also do laser procedures in some states.
Surgeries for more advanced cases
An ophthalmologist may need to use a different kind of surgery if other treatments don’t lower your eye pressure enough. Some of them are:
- Trabeculectomy – The eye surgeon creates a new pathway and a tiny bubble along the white part of your eye so more fluid can filter out.
- Drainage implant surgery – The surgeon places a small tube device (shunt) and a tiny plate in the eye to help more fluid drain out.
- Minimally invasive glaucoma surgery (MIGS) – The surgeon uses a smaller incision to implant a tiny device that helps fluid flow out.
LEARN MORE about the different surgeries used to treat glaucoma.
Frequently asked questions about glaucoma
How common is glaucoma?
Glaucoma is fairly common. In the United States, around 2.5% of adults over 40 have glaucoma, according to research by JAMA Ophthalmology. Between 3% and 5% of people worldwide have glaucoma, according to the Journal of Clinical Medicine.
Will I go blind if I have glaucoma?
It’s very unlikely that glaucoma will cause blindness if you keep up with treatment and routine eye exams.
If glaucoma isn’t treated or is diagnosed very late, vision loss usually starts as blind spots in your side vision worsen over time. Eventually, it can also harm the center of your vision.
There are many ways to slow or even stop this damage. Working closely with your eye doctor is the best way to protect your eyesight for the future.
Can you prevent glaucoma?
You can’t prevent glaucoma from starting, but you can help prevent vision loss by going to regular eye exams and following your eye doctor’s treatment plan.
Can glaucoma be cured completely?
Researchers haven’t found a cure for glaucoma yet. Once it develops, you usually have it for the rest of your life, but treatment can protect your eyesight.
How often should I get my eyes checked for glaucoma?
How often you need eye exams depends on your eyes, age, race and other risk factors.
- If you’re at high risk – Your eye doctor will probably recommend a comprehensive eye exam every one to two years. Some people need exams more often.
- If you aren’t at high risk – The American Optometric Association recommends all adults get a full eye exam every one to two years (every year if you’re 65 or older). This helps find hidden problems like glaucoma early on, so you can start treatment right away.
- If you already have glaucoma – Your doctor will probably want to see you at least twice a year to monitor your eyes and adjust treatment if needed.
Schedule an eye exam if it’s been a while since your last checkup. Your eye doctor will check for glaucoma and let you know how often you should come back.