Eylea injections: Treatment for wet AMD eye conditions
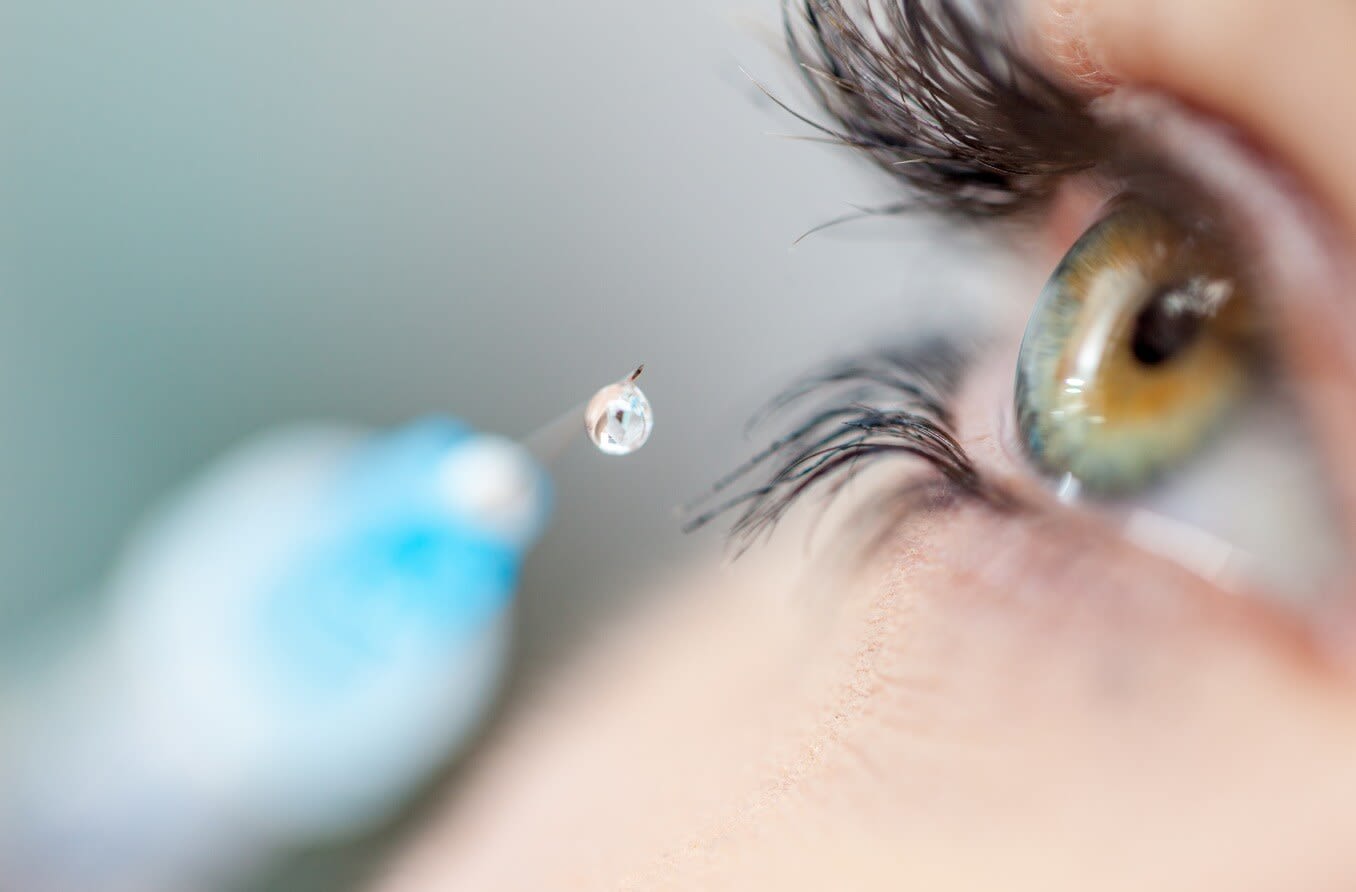
What is Eylea?
Eylea injections help slow or stop vision loss related to certain eye conditions by “drying the retina.” It works by reducing the fluid leaking into the macula. These conditions include diabetic retinopathy, macular edema and wet age-related macular degeneration (AMD).
AMD is a leading cause of vision loss in people 60 and older. There is no cure for wet AMD, but Eylea is a proven effective treatment.
Eylea is the brand name for the generic medication aflibercept. It is an FDA-approved prescription medicine administered by an ophthalmologist by injection into the eye.
Patients treated with Eylea may note improvements in their vision within three months. The medication has been clinically proven to help improve and maintain vision in patients with wet AMD for up to four years when treatment is continued.
How does Eylea work?
Eylea works by blocking a chemical called vascular endothelial growth factor (VEGF) that stimulates the growth of blood vessels.
High levels of VEGF in the eye can cause the growth of leaky, abnormal blood vessels which can swell and leak fluid into the macula, the small central part of the retina responsible for how clearly we see. This can lead to scarring of the macula resulting in decreased and distorted central vision.
Eylea is an anti-VEGF treatment. By blocking VEGF, Eylea helps to reduce the fluid leaking into the macula and the formation of new blood vessels. It slows the progression of wet AMD and can save and even improve vision.
Procedure
Eylea injection is performed as an outpatient procedure in an ophthalmologist’s office.
The doctor first cleans carefully around the eye to prevent infection.
They then use topical anesthetic drops on the eye to block pain.
A small device is placed around the eyelids to keep them open.
Once the eye is numb, the doctor uses a very thin needle to inject Eylea directly into the white part of the eye.
The medicine is absorbed by the vitreous humor, the gel-like substance that fills the back of the eyeball.
The patient may feel some pressure but no pain.
The entire procedure should take no more than 30 minutes.
In the first three months, Eylea’s manufacturer, Regeneron Pharmaceuticals, recommends a dosing schedule for wet AMD of once a week for four weeks, then one injection every eight weeks. Some patients may need monthly dosing after the first three months.
If you are treated with Eylea, your doctor decides the dosage and treatment schedule that’s best for you based on your medical condition and how you respond to treatment. They may combine other treatments with Eylea injection to help save your vision.
Expect your doctor to schedule periodic eye exams to check for side effects from the treatment and monitor your progress. It is important to keep all your follow-up and treatment appointments. If you miss one, contact your doctor as soon as possible to reschedule.
Conditions treated by Eylea
Eylea is approved by the FDA to treat four serious eye conditions that, if not treated, can cause vision loss. Eylea dries the retina by reducing swelling and leaking blood and other fluids.
Wet AMD
Abnormal blood vessels that grow under the retina cause wet AMD. Leaking blood and fluid kills or injures the cells in the macula that are light sensitive, creating a central blind spot or distortion in the person’s field of vision. This is the area that provides the fine detail needed for reading, driving and recognizing faces.
Wet AMD is not a painful condition, but it progresses quickly. It can permanently damage the macula and cause loss of vision.
Symptoms to watch for include:
Blurry or distorted areas in the center field of vision
Gray or black areas in the central field of vision if macular edema is present
Floaters, or spots floating in the field of vision
Straight lines that appear curved or wavy
Washed-out or dull colors
Objects that appear smaller than they are or different sizes in each eye
Difficulty distinguishing facial features
Need for more light when working within arm's length
Macular edema following retinal vein occlusion (MEfRVO)
Macular edema is a thickening of the macula due to swelling. Retinal vein occlusion is the blockage of a blood vessel in the retina, often by a blood clot. Fluid may leak into the macula, causing blurring or vision loss.
Symptoms of MEfRVO are:
Blurred central vision
Straight lines that look wavy
Decreased color vision
Vision loss
Diabetic retinopathy (DR)
Diabetic retinopathy is a complication of both Type 1 and Type 2 diabetes. It is the leading cause of new cases of blindness in people aged 20 to 74 in the United States.
DR is the most common reason for loss of vision in diabetics. When blood sugar levels are too high, it can lead to the retina not getting enough oxygen and nutrients. This is because small blood vessels in the eye swell and become blocked, depriving the retina of nourishment. In addition, new abnormal blood vessels can grow and leak into the retina.
Early symptoms of DR are subtle and may go unnoticed. As it progresses, it can cause blurred vision and floaters.
Diabetic macular edema (DME)
Diabetic macular edema is a complication of diabetic retinopathy. DME can happen at any time during the progression of DR.
DME is swelling of the macula from leaked fluid from damaged blood vessels. The swelling affects central vision, making it difficult to read and drive. Other symptoms are center vision blurriness, colors that appear washed out and straight lines that look wavy.
Risks of Eylea treatment
Every medical treatment has associated risks, and Eylea injection is no exception. You and your ophthalmologist should discuss what you can expect from the treatment and whether the potential risks outweigh the expected benefits.
Some risks of Eylea injections are:
Temporary increase in eye pressure
Blood clots, increasing risk of heart attack or stroke
Discuss these potential risks with your eye doctor prior to deciding on treatment.
Side effects of Eylea injections
Side effects are common with most medications and medical treatments. Whether mild or more serious, they are usually temporary.
Mild side effects associated with Eylea are:
Floaters
Moving spots in the field of vision
More serious side effects include:
Allergic reaction to Eylea
Double vision in the treated eye
Seeing colors as faded
Temporary changes in vision may occur after Eylea treatment and eye exams. Do not drive or use machinery until your vision recovers.
Let your doctor know if you experience any of these side effects or other issues after your Eylea treatment.
SEE RELATED: Lucentis vs. Avastin: A macular degeneration treatment controversy
Wet AMD: Anatomy outcomes. Regeneron Pharmaceuticals. August 2021.
Aflibercept (Intraocular Route). Mayo Clinic. February 2022.
Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: A prospective clinical trial. American Journal of Ophthalmology. June 2017.
Long-term outcomes of aflibercept treatment for neovascular age-related macular degeneration in a clinical setting. American Journal of Ophthalmology. December 2016.
Wet AMD. Regeneron Pharmaceuticals. May 2022.
New strategy for treating common retinal diseases shows promise. National Eye Institute. October 2020.
Anti-VEGF treatments. American Academy of Ophthalmology. March 2019.
What is Eylea? American Academy of Ophthalmology. April 2022.
Intravitreal injection. MedlinePlus. December 2020.
Dosing and administration. Regeneron Pharmaceuticals. May 2022.
Macular degeneration. MedlinePlus. April 2016.
Wet macular degeneration. Mayo Clinic. December 2020.
Macular degeneration. American Optometric Association. Accessed October 2022.
Macular edema and its connection to age-related macular degeneration. BrightFocus Foundation. August 2021.
Macular edema. National Eye Institute. August 2022.
Diabetic retinopathy. American Optometric Association. Accessed October 2022.
Diabetic retinopathy. Prevent Blindness. Accessed October 2022.
Diabetic retinopathy. National Eye Institute. July 2022.
Public health focus: Prevention of blindness associated with diabetic retinopathy. Centers for Disease Control and Prevention. May 2001.
Diabetic macular edema. Mayo Clinic. Accessed October 2022.
Diabetes-related macular edema. Prevent Blindness. Accessed October 2022.
MEfRVO. Regeneron Pharmaceuticals. June 2021.
Frequently asked questions. Regeneron Pharmaceuticals. May 2022.
Eylea (aflibercept). Medical News Today. June 2022.
Page published on Wednesday, November 9, 2022
Page updated on Tuesday, November 15, 2022
Medically reviewed on Monday, October 31, 2022






